This important announcement signals a vital new support tool in SA’s war against COVID-19.
The first approved COVID-19 antibody rapid test kits – authorised by South African Health Products Regulatory Authority (SAHPRA) – have finally arrived in the country, with Direct Retail Goods announcing the receipt of the first 10 000 units, and another 750 000 on order.
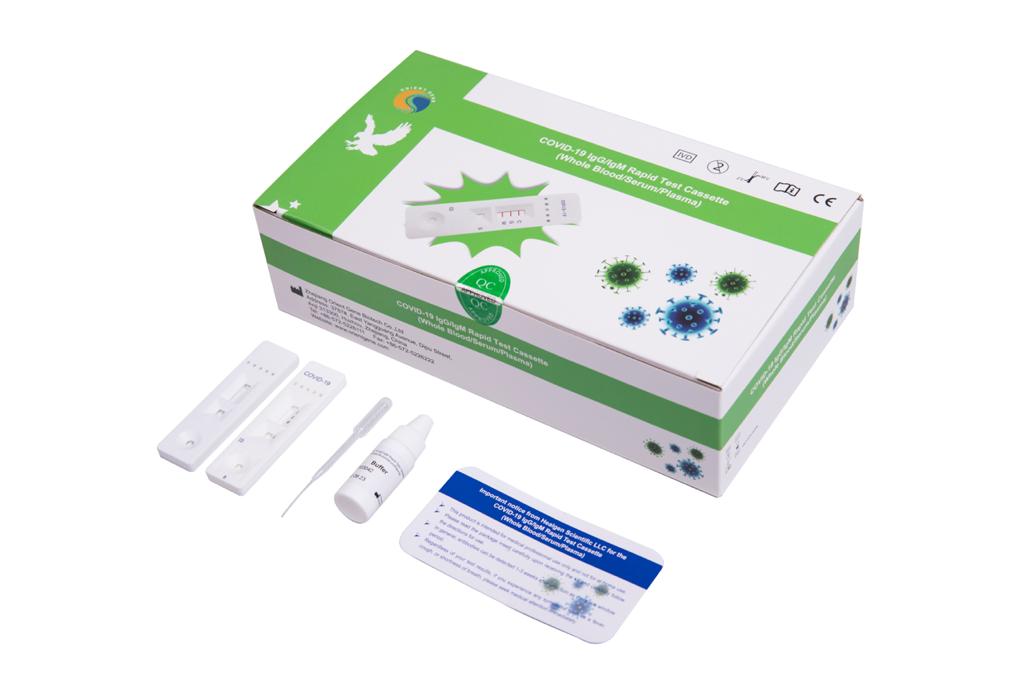
The single-prick SARS-CoV-2 IgG/IgM rapid test kits, made by Zheijiang Orient Gene Biotech, analyse blood to detect SARS-CoV-2 antibodies that are formed by the immune system in response to a past infection with the virus. They are useful in picking up evidence that a person had the virus even if they were asymptomatic, and were used effectively in a large COVID-19 serological survey in Spain.
With just a small number of approved and certified SAHPRA distributors, the news is welcome to the health industry, and the next step in assisting South Africa to reduce infection rates – which now exceeds 500 000 cases, over 9500 deaths, and approximately 8000 to 10000 new cases per day – by providing insight whether someone may have been infected without knowing.
Direct Retail Goods CEO, Graeme Pienaar, added, “The arrival of this stock finally signals an additional tool in our country’s war with coronavirus. With 750 000 units on order, we have commenced the process to secure further stock of these test kits, able to relieve the snowballing backlog of diagnostic COVID-19 tests. The factory’s manufacturing capacity is 2 million kits per day so this announcement may be the additional support that our frontline needs.”
In a 22 July communication to stakeholders, Boitumelo Semete-Makokotlela, Chief Executive Officer of SAHPRA, explained, “COVID-19 serological tests do not detect the virus itself. Instead, they detect the antibodies produced in response to an infection. Serological tests are not appropriate for clinical diagnosis of COVID-19. SAHPRA has, however, taken the position (md005) to authorise the COVID-19 serology tests that meet the target product profile (md007) under section 21 authorisation. It will be limited for use under the national testing protocol only and is recommended to supplement nucleic acid testing for the diagnosis of suspected COVID-19, to identify recent or remote past sars-CoV-2 infections, targeted cohort surveillance, community screening, specifically for serosurveys or hot spot tracing, population-level epidemiologic studies and surveillance programmes, identification of convalescent plasma donors, and as part of scientific research studies.”
“The moment the national test algorithm is released by the COVID-19 ministerial advisory committee, tests can be distributed,” he said. “The algorithm will tell us the appropriate place, how and when the tests can be used. Our trusted relationships with well-known pharmacies, clinics and healthcare professional chains will enable us to expedite the distribution of antibody test kit stock. Direct Retail Goods is incredibly excited to be leading the charge in South Africa,” Pienaar concluded.